

Introducing PROPEL
The PROPEL sinus stent is placed in the sinus after surgery to promote healing and reduce the need for additional post-operative procedures.1
To learn more about the benefits of PROPEL and safety information, visit MySinusitis.com.
Don’t Just Treat the Symptoms. Treat the Source.
Sinus surgery with PROPEL is clinically proven to provide relief by targeting inflammation, the primary characteristic of chronic sinusitis.2
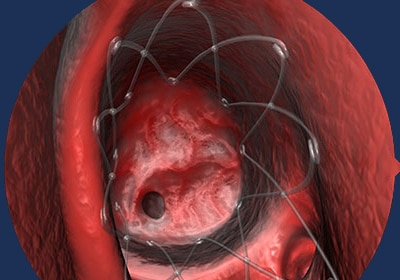
01 | OPENS
After sinus surgery, PROPEL is placed to help prop open the sinus.
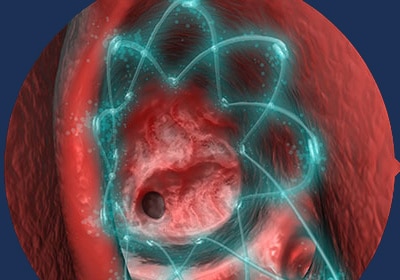
02 | DELIVERS
PROPEL delivers medicine directly to the sinus to reduce inflammation that could block the opening.
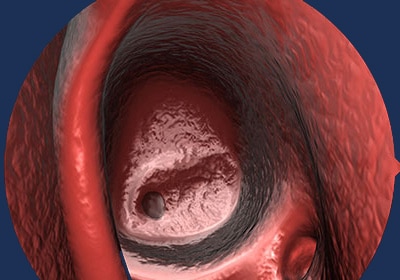
03 | DISSOLVES
As the sinus heals open, PROPEL is designed to dissolve within 45 days.
Testimonials
The videos below reflect individual responses to the PROPEL® sinus stent. The accounts given are genuine and documented. Each video represents a unique individual experience and does not provide any warranty or guarantee as to the response other people may have to PROPEL. The response other individuals have to PROPEL could be different. Responses can and do vary. See your physician for more information. Learn more about the risks associated with PROPEL at the bottom of this page.
Tinffany’s Story
1. Han JK, Marple BF, Smith TL et al. Int Forum Allergy Rhinol. 2012 2:271-279.
2. Forwith KD, Chandra RK, Yun PT, et al. ADVANCE: A multisite trial of bioabsorbable steroid-eluting sinus implants. Laryngoscope. 2011; 121:2473-2480.
The PROPEL sinus implants are intended for use following sinus surgery to maintain the sinus openings and to locally deliver a drug to the sinuses: PROPEL for use in the ethmoid sinus, PROPEL Mini for use in the ethmoid sinus and frontal sinus opening and PROPEL Contour for use in the frontal and maxillary sinus openings. The products are intended for use in patients ≥18 years of age. These products are not intended for people who are, allergic to the drug (mometasone furoate) or to certain polymers. Safety and effectiveness of the implant in pregnant or nursing females have not been studied. Risks may include, but are not limited to, pain/pressure, movement of the implant (within or out of the sinus), possible side effects of the drug, infection, and nose bleed. For more information on the risks and benefits of PROPEL sinus implants, please talk to your doctor. The FDA approved labeling can be found at www.IntersectENT.com. Rx only.
INTERSECT ENT and PROPEL are registered trademarks of Intersect ENT, Inc. in the United States and other countries. MySinusitis.com is a trademark of Intersect ENT, Inc.